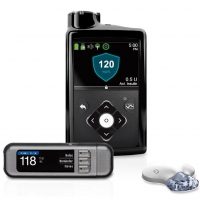
(Gray News) – The Food and Drug Administration has issued a Class I recall, the most serious type of recall, on certain Medtronic MiniMed 600 series insulin pumps used by thousands of individuals with Type 1 diabetes.
There have been over 26,000 complaints, 2,175 injuries and one death related to the insulin pumps, according to a statement released by the FDA.
The defect has to do with a retainer ring that does not lock in the insulin cartridges properly. The defective pumps provide an “over or under-delivery of insulin, which could then result in hypoglycemia or hyperglycemia,” according to a letter Medtronic sent to customers in November.
The recall includes over 322,000 devices.
“At Medtronic, patient safety is our top priority, and we are committed to delivering safe and effective therapies of the highest quality and reliability possible,” the letter said.
Individuals with a defected pump should contact Medtronic for a replacement.







